Clin Exp Dent Res.2021;7:772–785
Read Full Study
Abstract
Objectives : Clinical validation of a bioluminescence imaging system (Cis) as measured by the level of agreement between clinician visual and tactile assessment of carious lesion presence and activity and the presence/absence of elevated luminescence on a tooth surface determined from intraoral image mapping.
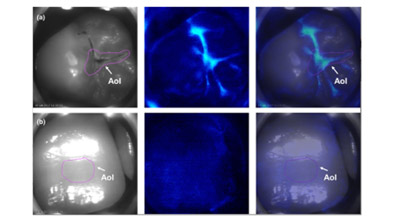
Materials and Methods: This was a regulatory clinical study designed in consultation with the FDA. The design was a prospective, five-investigator, nonrandomized, post-approval, clinical study utilizing the Cis to provide images of elevated calcium ion concentration (indicative of active demineralization) on tooth surfaces via use of a photoprotein. Imaged teeth were identified as “sound” or having “active lesions.” Images were scored independently for luminescence.
Results: A total of 110 participants aged 7–74 years were imaged. Of the 90 teeth assessed as “sound,” 88 were deemed to show no luminescence by the reviewing investigator, a negative percentage agreement of 97.8% (significantly >50% agreement [p < .0001]; one-sided 97.5% confidence interval [CI]: 0.9220). Of the 86 teeth initially assessed as having an “active lesion,” 78 were deemed to show luminescence by the reviewing investigator, a positive percentage agreement of 90.7% (significantly >50% agreement [p < .0001]; 97.5% CI: 0.8249). There were no patient-related adverse events.
Conclusions: Results show, with a high level of agreement, that Cis can differentiate tooth surfaces clinically identified as involving active enamel lesions (ICDAS code 2/3), from sound sites (biochemically equivalent to inactive lesions) and that the system is safe for clinical use.